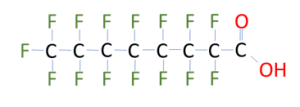UNDERSTAND WHAT PFAS ARE, THE CHEMISTRY BEHIND THEM, AND WHY THEY’RE KNOWN AS “FOREVER CHEMICALS”.

BY: BRAD LEWIS
The 2019 release of the film Dark Waters told the true story of the legal action taken by a farmer and an attorney to hold DuPont accountable for damages allegedly caused by perfluoroalkyl compounds, otherwise known as the forever chemicals. But even without this movie, perfluoroalkyl and polyfluoroalkyl substances (PFAS) compounds have been in the news. What are PFAS and why is there so much attention on them? PFAS are a family of diverse chemical compounds developed in the 1940s, that were found to be useful in a wide range of commercial products including water and stain repellent coatings on cookware, firefighting foams, and in industrial applications for cardboard coatings, and mist suppression for metal plating to name a few. Many industries include textile and leather manufactures, paper manufactures, metal plating industries, wire manufacturing, industrial surfactants, photo-lithography, airports, and firefighting, were quick to incorporate these compounds into their processes. So why are PFAS an evolving issue? The short answer is:
- Their widespread use in industrial and consumer products;
- Their suspected toxicity (very low proposed cleanup standards);
- Their persistence in the environment; and
- Their resistance to treatment technologies.
PFAS present a challenge to industry, regulators and scientists who are racing to understand the significance of these compounds in the environment. This is an emerging situation and work is proceeding on multiple fronts to address the concerns.
There are six things that must be considered when figuring out how to address PFAS:
- Evaluation of emerging toxicology data
- Evaluation of developing testing methods
- Evaluation of developing cleanup methods
- Developing regulations
- Understanding how prevalent these compounds are
- Under fate and transport in the environment
To learn more, read “What are PFAS compounds and how can we test for them?”
UNDERSTANDING THE CHEMICAL NATURE OF PFAS
Although there are thousands of PFAS compounds, at their core, they are characterized by having carbon and fluorine bonds, one of the strongest bonds in organic chemistry. These compounds come in various carbon chain lengths, which effect the fate and transport in the environment, their resonance time in the food chain, and ultimately the human body and their subsequent cleanup criteria. Once you understand the nomenclature, you can tell a lot from the compounds name. Two of the most manufactured PFAS compounds were perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA).
PFOS PF (Perfluoro=fully fluorinated carbons) O (Octa or 8 carbon chain) S (Sulfonic Acid)

C8HF17O3S
PFOA PF (Perfluoro=fully fluorinated carbons) O (Octa or 8 carbon chain) A (Carboxcylic Acid)
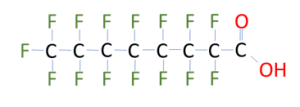
C8HF15O2
Like the compounds above, most PFAS compounds have a hydrophilic head (acid, alcohol, etc.) and a hydrophobic tail (carbon-fluoride chain). This dual nature of the compounds imparts much of the non-stick, water repellant, stain repellant properties that made them so useful in industrial applications. The carbon-fluoride bonds make PFAS compounds incredibly stable and resistant to breakdown. Hence the name “forever chemicals”. Most organic compounds would break down in a landfill, be degraded by natural bacteria, or be treated by a wastewater treatment plant, whereas PFAS compounds are generally resistant to these degradation processes. These compounds pass through most treatment systems intact to be discharged back into the environment. Their widespread use in products and their resistance to breakdown (or treatment) means that they may be widespread in the environment. While we do not know how prevalent these compounds are in the environment, we do know that wherever we look for PFAS, we are finding them.
Learn more about how federal and state governments are regulating PFAS for drycleaners and other small businesses.
The toxicological data indicates that low level concentrations of some PFAS compounds may affect liver function, impede immunological response, promote developmental effects, decrease fertility, increase hypertension and potentially cause testicular and kidney cancers. The toxicity of PFOS and PFOA are probably the most studied and most understood. However, the chemical toxicity for a majority of PFAS and GenX (PFAS replacement chemicals) compounds have not been studied. Early indications are that most cleanup levels for these compounds will be in the one part per trillion-part (ppt) range, which is equivalent to one ping pong ball in a sea of a trillion ping pong balls.
If you have any questions about PFAS, including the chemistry, regulations, remediation or how to fund PFAS cleanup and legal defense, please contact us.
PFAS SAMPLING AND TESTING
The low cleanup levels come with their own set of challenges. How do you test for one part per trillion (ppt) of a compound in a water sample when the notebook, raincoat or sunscreen your personnel is wearing was manufactured with PFAs? The answer is you must be careful and beware of false positives. Currently there are two validated USEPA analytical methods SW 846 Method 537.1 and Method 533, both of which are for drinking water. There are no adopted USEPA methods for non-drinking water and soil, although modified methods do exist. Analytical methods still need to be developed and commercial laboratories will need to invest in equipment and processes to analyze this emerging contaminant. Currently there are a limited number of laboratories qualified to test for PFAs.
REGULATORY ENVIRONMENT FOR PFAS
The regulatory environment for PFAs is a patchwork, as some states have been quick to establish a regulatory framework while most are waiting for the United States Environmental Protection Agency (USEPA) to take the lead. Several states have gotten out in front of the USEPA and have either tested water supply wells like Michigan or are beginning to require contaminated sites to include PFAs on their analyte lists. To date, the USEPA has concentrated on two of the most manufactured PFA compounds, PFOS and PFOA. While the USEPA is concentrating on a smaller subset of the PFA compounds, some states like Wisconsin are requiring evaluation of a much larger number (16) of PFA compounds at sites.
The U.S. EPA currently has a task group which is evaluating the PFAs issue. One of the first hurdles of this task group is to set drinking water maximum contaminate levels (MCLs) that will apply to suppliers of potable water. These drinking water standards are expected to be published in 2021 or 2022. This will mean that any supplier of water from groundwater or surface water source will begin testing for these compounds and if found will need to treat them. Similar testing of water supply wells in some states have led to the identification of PFA release sites.
More important to you perhaps is what do you do if a regulating entity asks you to sample your site for PFAs? Before you respond, there are some questions you need to ask yourself.
- Under what authority is the regulator requiring PFAs testing?
- Specifically, which of the PFAs chemicals are you required to test for?
- How low is the concentration we need to look for?
- If I generate a concentration for a PFAs compound to what standards will it be compared?
What you should not do is rush into a sampling event without much consideration. Additional information regarding regulatory framework for PFAS will be provided in upcoming Blog posts.
PFAS CLEANUPS
Traditionally, environmental cleanups have involved either the physical removal of the contaminant from the environment, immobilization of contaminants and/or the biologically or chemical destruction of the contaminant in place.
Physical Contaminant Removal
PFAS can be remediated by physical removal. This entails excavation of soil and/or the pumping of groundwater followed by their concentration onto various sorbent materials. Both processes create impacted wastes that still require disposal, storage or destruction.
While excavation is an effective way of removing soil contaminant mass, its use is often limited by depth of impacts, presence of groundwater, presence of building structures, cost effectiveness, and potential long-term liability for waste generated. Wastes placed into modern lined landfills typically decompose and combine with water to form leachate. This leachate migrates to collection systems in the bottom of the landfills, which is treated and then ultimately discharged back into the environment. Based on their stability in the environment, PFAS have the potential to migrate untreated through the landfill and through the wastewater treatment plant to be discharged back into the environment. As landfills become more cognizant of the issues surrounding PFAS there is some question as to their willingness/ability to except these waste streams. Ultimately these wastes constitute a potential long-term liability unless the contaminant is ultimately destroyed. High temperature incineration of PFAS derived wastes is one way of permanently destroying these compounds; however, there are issues of the high costs of incineration and potential for incomplete combustion of these compounds.
One industry leader TRS Group has a patent for a thermal remediation system that heats soil (in situ or ex situ) to between 350 to 400 degrees C, which drives off PFA compounds in the vapor phase. The vapor phase is recovered and then either thermally oxidized or the PFAs are condensed and treated in the aqueous phase[1].
Contaminant Immobilization
Sometimes the goal of a remediation is less about reduction of contaminants and more about ensuring that contaminants do not migrate to potential sensitive receptors. The concept of immobilizing contaminants in-place to prevent migration is nothing new and has been employed for years at sites with metal contaminants. One method that is being studied for PFAs is the injection of colloidal activated carbon (CAC) into aquifers to provide sites for PFA sorption. While the sorption mitigates the migration of contaminants to downgradient receptors, actual contaminant mass remains unaffected. The longevity of these subsurface sorption barriers and their ability to hold the PFAs indefinitely, is currently being studied.
Biological or Chemical Destruction
For cleanup of sites impacted with petroleum and/or chlorinated solvents, for years scientists and engineers have made full use of the fact that these organic compounds, are subject to both abiotic (chemical) and biotic (bacterial, fungal, etc.) mediated breakdown into less toxic byproducts. These processes, either with or without augmentation, are employed to reduce contaminant mass in place, without the need for physical removal and ex situ treatment. These remediation techniques have become popular since they are cost effective, less disruptive to infrastructure, and do not generate legacy wastes. By their nature, PFA compounds appear to be resistant to these processes; however, the jury is still out and this an active field of research. Currently these methods are in the realm of college research papers and experimental cleanups at defense and superfund sites and are not options for most sites.
Conclusion
What should you do if you are potential responsible party in a PFA release? First and foremost, take a deep breath. It is important that you choose a consultant like EnviroForensics who will carefully listen to all the details of your situation and consider your potential liabilities, regulatory, financial and investigation options. With the science and regulations rapidly evolving around PFA issues, it is important to know the landscape before charging ahead. One thing is very clear however, there will be greater and greater regulation of these chemicals and their subsequent release to the environment. Hopefully, the science and technology to address these unique chemicals can developed to meet these challenges. Stay tuned for additional articles on this subject.
Learn more about our environmental investigation and remediation services.
 Brad Lewis, CHMM, Principal Scientist at EnviroForensics
Brad Lewis, CHMM, Principal Scientist at EnviroForensics
Brad Lewis is a detailed-oriented and collaborative leader with 30+ years of environmental consulting experience that covers a variety of projects ranging from due diligence, environmental compliance, landfill, Brownfields, underground storage tank, and chlorinated hydrocarbon investigations and cleanups. As Principal Scientist, he oversees investigations and cleanups. He helps project teams set the technical and regulatory strategies that will meet their client’s goals. Lewis has implemented many innovative site investigation strategies including the use of down-hole sensing equipment, mobile laboratory, and an immunoassay to characterize sites.
He has consulted on many high-profile projects dealing with petroleum hydrocarbons, polychlorinated biphenyls, hexavalent chromium, chlorinated solvents, bedrock impacts, vapor intrusion investigations, and vapor mitigation.
[1] Crownover E, Oberle D, Kluger M, Heron . Perfluoroalkyl and polyfluoroalkyl substances thermal desorption evaluation. Remediation 2019;29:77-81

 Dru Shields, Director of Drycleaner Accounts
Dru Shields, Director of Drycleaner Accounts